Review Autoclaves are designed to kill which of the following heat-resistant microbes?
Kinh Nghiệm Hướng dẫn Autoclaves are designed to kill which of the following heat-resistant microbes? Mới Nhất
Hoàng Đại Thắng đang tìm kiếm từ khóa Autoclaves are designed to kill which of the following heat-resistant microbes? được Cập Nhật vào lúc : 2022-12-10 09:50:08 . Với phương châm chia sẻ Bí quyết về trong nội dung bài viết một cách Chi Tiết Mới Nhất. Nếu sau khi Read nội dung bài viết vẫn ko hiểu thì hoàn toàn có thể lại Comment ở cuối bài để Ad lý giải và hướng dẫn lại nha.Steam sterilization is an important process, one that is performed in every laboratory. In this article, we will explore the history of steam sterilization, how a sterilizer works, and emerging trends in sterilizer design.
Nội dung chính Show- Table of ContentsAn Introduction to Steam SterilizationTerminologyHistory of the AutoclaveMeet the People Behind Our Autoclaves >>>What is Sterility?How Does an Autoclave Work?General ProcessCritical Components of an AutoclaveSterilization CyclesEmerging Autoclave TrendsYour Go-To Source for All Things Autoclave-RelatedFrequently Asked Questions17 Questions to Ask Before Buying Your Next AutoclaveWhich of the following are the standard conditions for an autoclave?Which of the following items are typically irradiated in order to kill microbes?What are the standard conditions for an autoclave quizlet?How does Pascalization control microbial growth?
Table of Contents
- An Introduction to Steam Sterilization
- TerminologyHistory of the AutoclaveWhy Steam?What is Sterility?
- General Process
- Purge PhaseExposure (Sterilization) PhaseExhaust Phase
- VesselControl SystemThermostatic TrapSafety ValveWastewater Cooling MechanismVacuum Systems (If Applicable)Steam Generator (If Applicable)
An Introduction to Steam Sterilization
Terminology
The terms steam sterilizer and autoclave are synonymous and can be used interchangeably. That said, autoclave is often used in laboratory settings, while sterilizer is more commonly heard in hospitals or pharmaceutical settings.

Autoclaves use steam heat to kill any microbial life that may be present on a contaminated load. A load — also known as goods — is considered sterile once it has undergone a full sterilization cycle. Once a load is sterile, it can be used without fear of introducing foreign microorganisms into a sensitive environment, such as a laboratory, hospital operating room, food production facility, and so on. Different types of goods must be sterilized for different lengths of time and different temperatures. Some autoclaves include additional features, such as vacuum functions, special cycles, and integral electric boilers.
History of the Autoclave
Dr. Charles Chamberland invented the autoclave in 1879, but the concept of using steam in an enclosed space in order to prevent sickness has existed in some form or other since 1679.
The principles and methods for sterilization have remained largely unchanged for the past 150 years. In fact, most major advancements in autoclave technology since 1879 have revolved around sterilization process monitoring, autoclave safety, and sterilization cycle creation rather than alterations to the sterilization process.
Meet the People Behind Our Autoclaves >>>
Why Steam?
In order to kill a cell with heat, its temperature must be raised to a degree which the proteins in the cell wall break down and coagulate. Steam is a very efficient medium for heat transference, which makes it an excellent way to destroy microbes. Air, on the other hand, is a very inefficient way to transfer heat/energy because of a concept known as the heat of vaporization.
It requires 80 kilocalories (kcal) of heat energy to bring one liter of water to its boiling point (100˚C). It would require 540 kcal to convert that liter of water into steam, which means that steam 100˚C contains seven times as much energy as water 100˚C.
That energy is what makes steam so much more efficient destroying microorganisms. When steam encounters a cooler object, it condenses into water. Then, it transfers all of the energy that was used to boil the water directly into the water. This process heats up the cells far more efficiently than air similar temperatures. This is why we use steam to achieve sterility.
What is Sterility?
Most people have a working understanding that sterile goods are không lấy phí of microorganisms and are, therefore, safe to use in medical, food production, research or other settings in which the presence of germs would be a significant safety hazard or detriment.
Exactly how many microorganisms will be left alive over time a fixed temperature is expressed as a probabilistic logarithmic curve — a function that approaches but never reaches zero (see Figure 1).

Figure 1
As the function approaches zero, one would typically choose a level of confidence — called the Sterility Assurance Level (SAL) — for the odds that the last microorganism present will survive. Contrary to popular belief, sterilization is not binary where something is either sterile or non-sterile. Sterilization is a statistical sự kiện characterized by this confidence factor (SAL). The general standard for SAL is 10-6, or a one-in-a-million chance of a single viable microorganism surviving. How long sterilization takes depends on the set temperature and SAL level desired; higher temperatures will achieve sterility faster.
As the function approaches zero, one would typically choose a level of confidence — called the Sterility Assurance Level (SAL) — for the odds that the last microorganism present will survive. Contrary to popular belief, sterilization is not binary where something is either sterile or non-sterile. Sterilization is a statistical sự kiện characterized by this confidence factor (SAL) . The general standard for SAL is 10-6, or a one-in-a-million chance of a single viable microorganism surviving. How long sterilization takes depends on the set temperature and SAL level desired; higher temperatures will achieve sterility faster.
How Does an Autoclave Work?
General Process
Whether it’s a small tabletop unit or a room-sized bulk unit, all autoclaves operate using principles similar to those of a common kitchen pressure cooker — that is, the door is locked to form a sealed chamber, and all air within that chamber is replaced by steam. The steam is then pressurized in order to bring it to the desired sterilization for the desired duration. Once the cycle is complete, the steam is exhausted, and goods can be removed.
For a more detailed explanation of the various phases of a sterilization cycle, please refer to the list and image (Figure 2) shown below:
1. Purge Phase: Steam flows through the sterilizer and starts to displace the air; temperature and pressure ramp slightly to a continuous flow purge.
2. Exposure (Sterilization) Phase: During this phase, the autoclave’s control system is programmed to close the exhaust valve, thereby causing the interior temperature and pressure to increase to the desired setpoint. The program then maintains the desired temperature (dwells) until the desired time is reached.
3. Exhaust Phase: Pressure is released from the chamber through an exhaust valve and the interior is restored to an ambient pressure (though contents remain relatively hot).
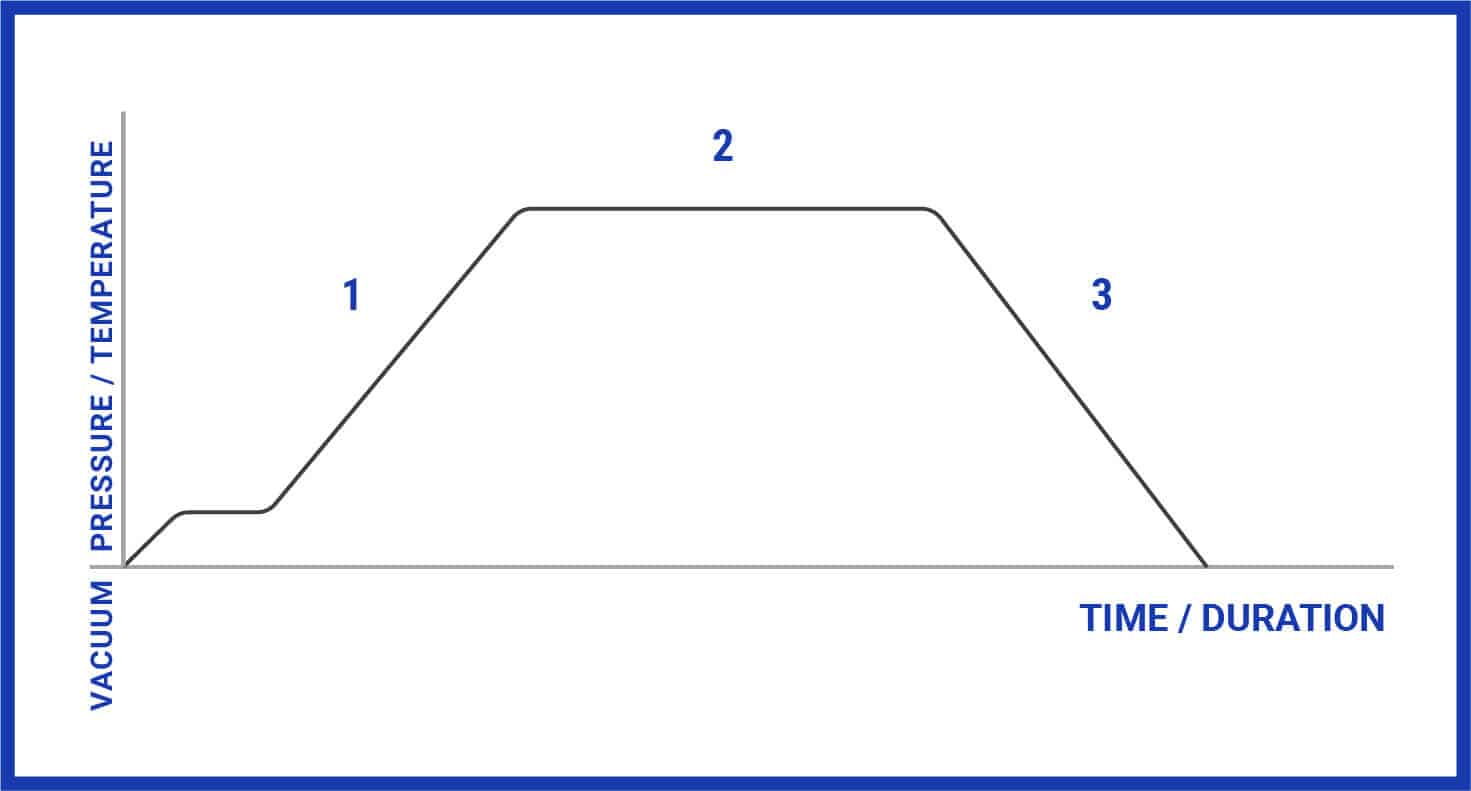
Figure 2
Critical Components of an Autoclave
The typical laboratory autoclave is comprised of the following components (Figure 3):

Figure 3
1. Vessel
The vessel is the main body toàn thân of the autoclave and consists of an inner chamber and an outer jacket. Laboratory and hospital autoclaves are constructed with “jacketed” chambers (see Figure 4), where the jacket is filled with steam, reducing the time that it takes to complete a sterilization cycle and reducing condensation within the chamber. A vessel designed and manufactured with a full jacket is superior to that of a partial jacket or blanketed jacket for the following reasons: a full jacket improves temperature uniformity within the chamber, it reduces the likelihood of wet packs, and helps minimize wet steam, which is not good for sterilization.
In the United States, every autoclave vessel is inspected and tagged with an American Society of Mechanical Engineers (ASME) nameplate that includes a National Board number. Manufacturers are required to hydrostatically test each vessel and apply an ASME nameplate before putting an autoclave into use. This inspection and the ASME nameplate are key indicators of a properly functioning autoclave.
Laboratory and hospital autoclave vessels can vary in size, from 100L to 3,000L, and are typically constructed from 316L stainless steel. Inner chambers are either 316L or nickel-clad, and outer jackets are made of 316L, 304L stainless steel, or carbon steel.
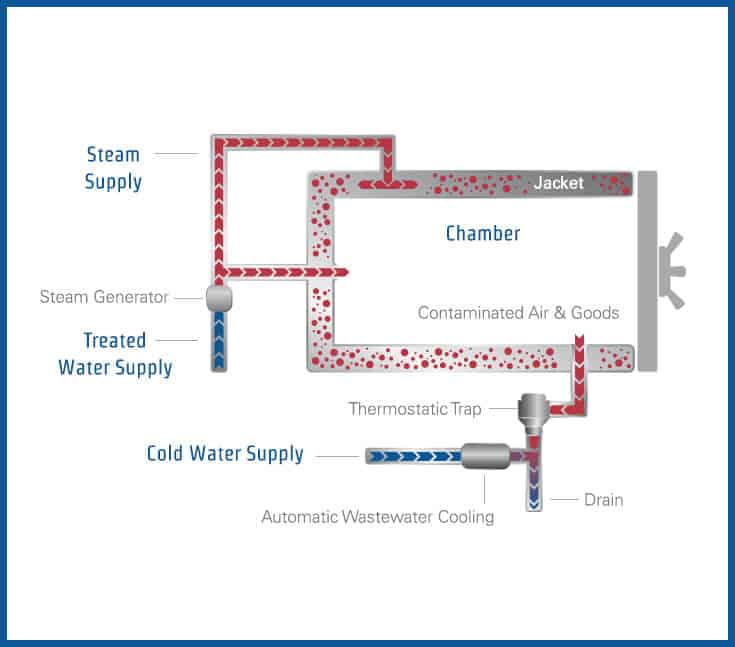
Figure 4
2. Control System
All modern autoclaves are equipped with a controller interface, not unlike what you would find on a microwave or oven. That said, autoclave control systems tend to be a bit more sophisticated than those of household appliances. A sterilization cycle follows a preprogrammed software formula that opens and closes valves and other components in a specific sequence. Therefore, all autoclaves require some form of control system, whether it’s as simple as a “push button” system with a microprocessor or as complex as a programmable logic controller with color touch screen.
3. Thermostatic Trap
All autoclaves feature some form of thermostatic trap or steam trap, a device designed to allow air and water (condensate) to escape from the chamber. Although a steam delivery system/steam autoclave might use a variety of traps, they all perform the same basic function: removing condensate while preventing the passage of dry steam. Most often, steam traps are temperature-sensitive valves that close when heated past a certain setpoint. Thermostatic traps are a critical component of any well-designed autoclave.
4. Safety Valve
All autoclaves operate under elevated pressure (14–45 pound-force per square inch gauge) and must therefore be manufactured with an incredibly robust construction and fitted with a number of safety features and devices to ensure they present no danger to users. One of these safety devices is the safety valve, which is the final fail-safe device for the pressure vessel should all electronic controls fail. It is imperative that the safety valve be inspected, tested, and verified to be in proper working condition based on the recommendations of the sterilizer and/or valve manufacturer, as well as local inspection and insurance agencies.
5. Waste-Water Cooling Mechanism
Many autoclaves are equipped with a system to cool effluent (air, steam, and condensate) before it enters the drain piping. Many municipalities and buildings do not allow effluent above 140˚F to enter the floor drain. In order to avoid damage to the facility’s drain piping, the steam must be cooled before it can be sent down the drain. The simplest method for cooling this steam is to mix it with additional cold tap water, but the amount of water required can cause an autoclave to be a major contributor to a building’s water usage. Some autoclaves come equipped with systems designed to reduce, or even eliminate, water consumption.
6. Vacuum System (if applicable)
Vacuum System (If Applicable): In order to ensure proper sterilization, it’s vital that all air inside the autoclave chamber be replaced with steam. Certain commonly sterilized goods — especially porous materials, such as animal bedding or cloth or containers with small openings, such as flasks or goods in bags — tend to retain air pockets. If an air pocket is present during the cycle, any microorganisms within that pocket will survive, and the goods will not be sterile.
For this reason, many sterilizers will include a vacuum system. This not only enables the user to forcibly remove air by using a vacuum on the chamber before a cycle (known as pre-vacuum), is also enables them to use a vacuum after the cycle (known as post-vacuum) to remove any steam that remains in the chamber and to dry off the goods inside the autoclave.
7. Steam Generator (if applicable)
A central “house” boiler is the most common steam source for an autoclave. However, if house steam is unavailable or insufficient for the autoclave, one must resort to using an electric steam generator, also known as a boiler. These boilers typically sit underneath the autoclave chamber and utilize electric heating elements to heat water and generate steam.
Need Help Choosing a Steam Source for Your Autoclave? Check This Out >>>
To learn more about autoclaves, check out our video here:
Sterilization Cycles
There are, in general, four standard sterilization cycles: gravity, pre-vacuum, liquids, and flash (also known as immediate use). The chart shown below explains these cycles in greater detail.
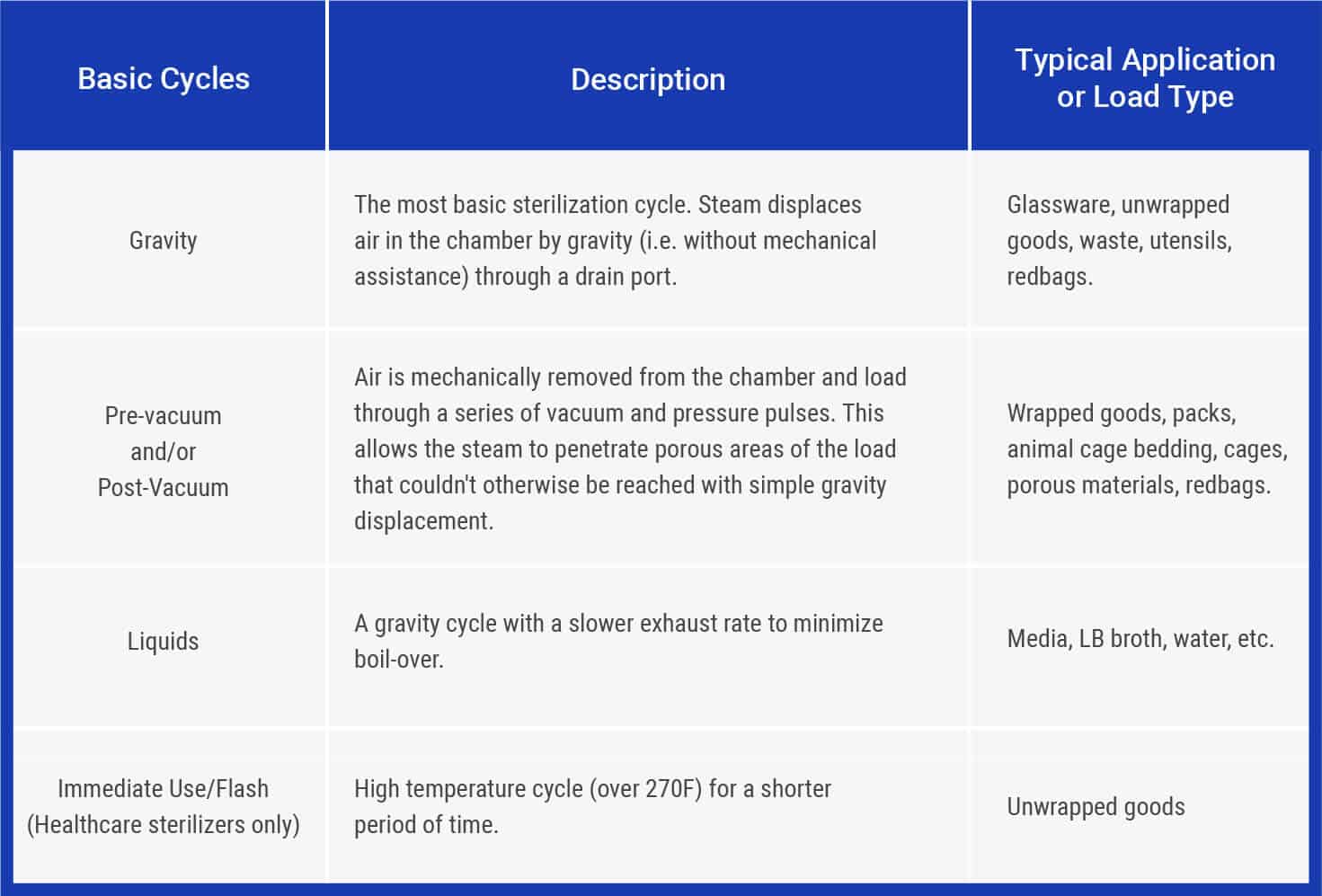
Some autoclaves also have the ability to perform specialty cycles designed to avoid damage to delicate goods that need to be sterilized but would be damaged or destroyed by the rapid changes in temperature and pressure in a normal cycle. These specialty cycles include much longer cycles lower temperatures, steam-air mix cycles with special pressure controls to avoid breaking sealed test tubes, and cycles that use special instrumentation to ensure full sterilization temperature is achieved.
Here’s What You Need to Know About Steam Sterilization Cycles >>>
Emerging Autoclave Trends
Autoclaves may be considered ancient devices by the standards of modern science, but this does not mean that autoclaves lack innovation, especially when it comes to controls, cloud connectivity, and ecological impact.
As mentioned earlier, autoclave controls have advanced greatly in the age of computers, progressing from manual controls and simple timers to computer automation that minimizes or eliminates entirely the need for user input. Computerized controls have also led to advances in data control, record keeping, and remote monitoring via mobile devices. Autoclaves with automatic printers that record data for the purpose of verifying successful sterilization have now been replaced by new autoclaves that connect to the cloud to store cycle records on the internet.
Another trend in autoclave design is sustainability. Autoclaves are a major source of water and energy consumption, both in laboratories and hospitals. In recognition of this, many manufacturers have found innovative ways to reduce autoclaves’ environmental impact. Green autoclaves that reduce or even fully recycle the water consumed by a sterilizer — in some cases, from 1,500 gallons a day to less than one gallon a day — are critical to create an environmentally friendly laboratory. Control systems that automatically turn the autoclave when not in use can also significantly reduce energy use — in some cases, from 80 kilowatt-hours per day to 20 kilowatt-hours per day.
Your Go-To Source for All Things Autoclave-Related
Whether you’re using an autoclave to sterilize medical or laboratory equipment, it’s important that you develop a keen understanding of the sterilization process — both how it works today and how it is changing.
Ask These Key Questions Before Buying Your Next Autoclave >>>
Consolidated Sterilizer Systems has a rich heritage in the steam sterilization industry, with over 75 years of experience. We strive to deliver manufacturing excellence and are committed to supplying high quality, high performance sterilization and distillation solutions. If you’re interested in learning more about the steam sterilization process, or have other autoclave-related questions, contact us today.
Frequently Asked Questions
Q.: How does an autoclave work?
A: Autoclaves use extreme heat in the form of pressurized steam in order to sterilize goods. Similar to a pressure cooker, an autoclave uses a locked door to create a sealed chamber. The air within that chamber is then replaced by steam, which is pressurized until the goods within the chamber have been sufficiently sterilized.
Q.: How does autoclaving kill bacteria?
A: Autoclaves use steam heat to raise temperatures to such a degree that proteins within the cell walls of a microbe break down and begin to coagulate, thereby killing the bacterium and achieving sterilization.
Q.: Why is autoclaving items better for sterilization purposes than boiling them?
A: Steam is a very efficient medium for heat transference. As a result, you can achieve higher temperatures using steam than boiling water, which makes it a more effective method of killing bacteria and other microorganisms.
Q.: How long does it take an autoclave to sterilize goods?
A: How long it takes to sterilize a load depends entirely on the content of the load, the set temperature of the autoclave, and the Sterility Assurance Level desired. Generally speaking, the higher the temperature, the faster a load will achieve sterility.
Q.: What temperature(s) can an autoclave reach?
A: Autoclaves are typically designed to reach temperatures between 250°F and 275°F (121°C and 135°C).
Q.: What are the phases of sterilization?
A: The general process for using an autoclave to sterilize goods breaks down into three basic phases:
The purge phase, during which steam displaces air within the autoclave chamber and temperature and pressure steadily increases
The exposure (sterilization) phase, during which the autoclave’s control program closes the exhaust valve, causing the autoclave’s interior temperature and pressure to increase to the desired setpoint, and stay there for the desired amount of time
The exhaust phase, during which pressure is released from the chamber via the exhaust valve, and the interior is restored to ambient pressure
Q.: How long do items stay sterile after autoclaving?
A: It depends entirely on how items are packaged after sterilization. Generally speaking, items should be re-sterilized after each use, but items packaged in double-wrap linen packs or an inner layer of paper and outer layer of plastic have been proven to remain sterile for up to 96 weeks.
Q.: What are the different types of autoclaves?
A: The two most common types of steam sterilizers are gravity displacement autoclaves and high-speed prevacuum autoclaves. Both types of autoclave come in a variety of shapes and sizes, ranging from tabletop units to room-sized bulk units, with a vast array of customization options, such as vertical sliding doors, double-door pass-thru chambers, and stackable dual chambers.
Article Sources
Centers for Disease Control and Prevention, “Steam Sterilization, https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/steam.html.”Science History Institute, “Dr. [Charles] Chamberland, https://digital.sciencehistory.org/works/sgm3742.”Consolidated Sterilizer Systems, “The Definitive Guide to Steam Sterilization Cycles, https://consteril.com/steam-sterilization-cycles-guide/.”Science.org, “Cell-wide analysis of protein thermal unfolding reveals determinants of thermostability, https://www.science.org/doi/10.1126/science.aai7825.”Science Direct, “Heat of Vaporization, https://www.sciencedirect.com/topics/earth-and-planetary-sciences/heat-of-vaporization.”Consolidated Sterilizer Systems, “Decontamination, Disinfection & Sterilization: What You Need to Know, https://consteril.com/disinfection-vs-sterilization/.”Science Direct, “Sterility Assurance Level, https://www.sciencedirect.com/topics/engineering/sterility-assurance-level.”PubMed, “The limits of sterility assurance, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2831250.”Consolidated Sterilizer Systems, “A Guide to Autoclave Steam Sources, https://consteril.com/products/sterilizers/help-me-choose/steam-source/.”The American Society of Mechanical Engineers, “Homepage, https://www.asme.org/.”Consolidated Sterilizer Systems, “6 Important Questions to Ask When Purchasing a Refurbished Autoclave, https://consteril.com/6-important-questions-when-purchasing-refurbished-autoclave/.”AccuTEST Systems, “Best Practices for Pressure Relief Valve Testing, https://www.accutestsystems.com/best-practices-for-pressure-relief-valve-testing/.”Water & Wastes Digest, “What Is Effluent?, https://www.wwdmag.com/wastewater-treatment/what-effluent.”HPAC Engineering, “The Importance of Drain-Water Tempering, https://www.hpac.com/archive/article/20927153/the-importance-of-drainwater-tempering.”Consolidated Sterilizer Systems, “Green Technology for Reducing Sterilizer Water Consumption, https://consteril.com/products/smart-options/water-saving-systems/.”Consolidated Sterilizer Systems, “Steam Sterilization Cycles Part 1: Gravity vs. Vacuum, https://consteril.com/steam-sterilization-cycles-part-1-gravity-vs-vacuum/.”Consolidated Sterilizer Systems, “Steam Sterilization Cycles Part 5: Low Temperature Cycle, https://consteril.com/steam-sterilization-cycles-part-5-low-temperature-cycle/.”Consolidated Sterilizer Systems, “Steam Sterilization Cycles Part 3: Steam-Air-Mix Cycle, https://consteril.com/steam-sterilization-cycles-part-3-steam-air-mix-cycle/.”Consolidated Sterilizer Systems, “Why to Autoclave Liquids with a Load Probe, https://consteril.com/laboratory-autoclave-load-probe/.”IBM, “What is automation?, https://www.ibm.com/topics/automation.”Consolidated Sterilizer Systems, “Consolidated Sterilizer Systems Unveils New Autoclave Sustainability Features, https://consteril.com/consolidated-sterilizer-systems-unveils-new-autoclave-sustainability-features/.”PubMed, “Steam sterilsation’s energy and water footprint, https://pubmed.ncbi.nlm.nih.gov/27075773/.”Consolidated Sterilizer Systems, “Celebrating 75 Years of Consolidated Sterilizer Systems, https://consteril.com/consolidated-celebrates-75-years/.”
Amit Gupta
Amit has over 15 years of experience engineering multi-disciplinary systems and brings his passion for engineering, innovation and quality to his work every day. Prior to joining Consolidated Sterilizer Systems in 2007, Amit worked on robotics the Charles Stark Draper Laboratories. Additionally, he designed medical devices and scientific equipment such as novel catheters and DNA hybridization chambers for microarrays. A true engineer heart, Amit enjoys breaking things just to figure out how to fix them; his love of tinkering even extends to assembling IKEA furniture. He graduated from the University of Pennsylvania with a B.S. in Mechanical Engineering and from MIT with an M.S. in Mechanical Engineering.
17 Questions to Ask Before Buying Your Next Autoclave
We created this 17-question eBook as a framework to help you explore and discover the exact type of autoclave best suited to your needs.
Which of the following are the standard conditions for an autoclave?
Autoclaves use saturated steam under pressure of approximately 15 pounds per square inch to achieve a chamber temperature of least 250°F (121°C) for a prescribed time—usually 30–60 minutes.Which of the following items are typically irradiated in order to kill microbes?
Answer and Explanation: Radiation sterilization is used for eliminating microorganisms during tissue allografting for the sterilization of tissue allografts such as skin, cartilage, and, heart valves. It is also used for the sterilization of cured meats by the destruction of food-spoilage microorganisms.What are the standard conditions for an autoclave quizlet?
Standard autoclave conditions (121°C, 15 pounds of pressure, 15 minutes) will sterilize which two of the following? Color change on autoclave tape indicates that _______.How does Pascalization control microbial growth?
Pascalization, bridgmanization, high pressure processing (HPP) or high hydrostatic pressure (HHP) processing is a method of preserving and sterilizing food, in which a product is processed under very high pressure, leading to the inactivation of certain microorganisms and enzymes in the food. Tải thêm tài liệu liên quan đến nội dung bài viết Autoclaves are designed to kill which of the following heat-resistant microbes?
Post a Comment